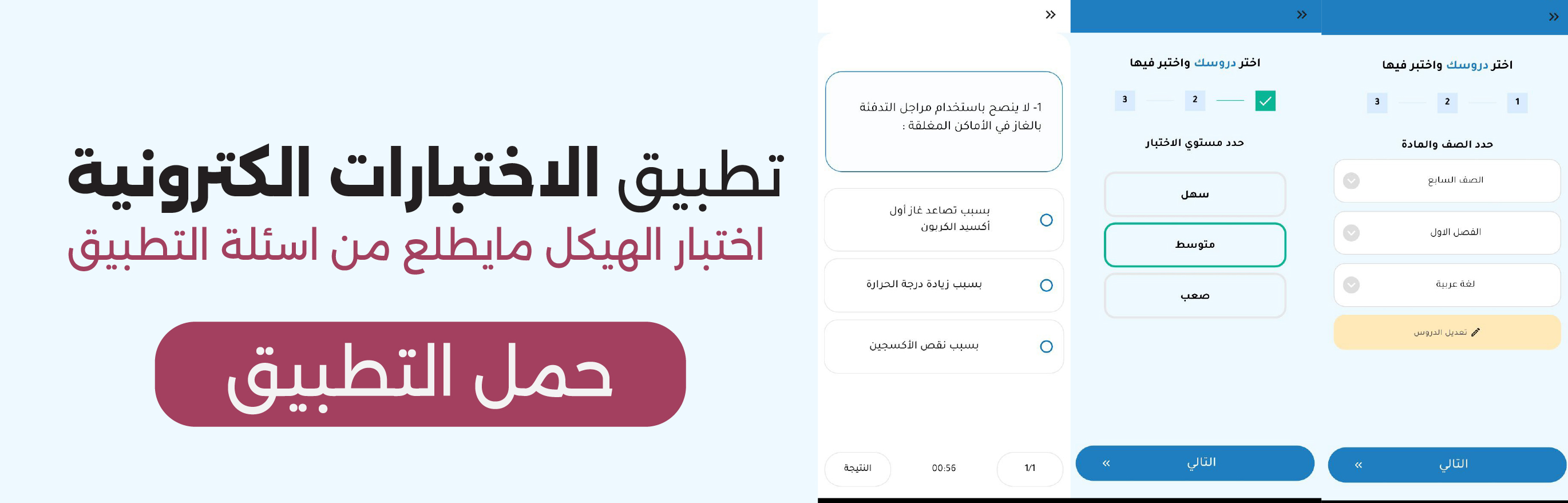
Science
Summary & Practice Sheets
Grade 9 – Biology
Chemistry in Biology Cellular Structure and Function Digestive and Endocrine system
Atoms, Elements, and Compounds
Atoms are composed of three smaller subatomic particles: protons, neutrons and electrons.
An element is a pure substance that is made of only one type of atom. It cannot be broken down into other substances by any physical or chemical means.
A compound is a pure substance that is made of two or more different types of atoms. It can be broken down into simpler compounds or elements by chemical means.
Radioactive isotopes have unstable nuclei. Their nuclei decay, or breakdown, over time and release energy.
PROPERTIES OF COMPOUNDS
FIXED RATIO
Pure water is always made up of 2 hydrogen atoms and 1 oxygen atom.
BREAK DOWN
In electrolysis, the passage of an electrical current through water will decompose water into hydrogen and oxygen gases.
UNIQUE PROPERTIES
Hydrogen and oxygen are gases when these elements combine they form water, a liquid.
Chemical Bonds
Elements combine together to become chemically stable. The electrons in the outer energy level of an atom can interact with the electrons in the outer energy level of other atoms. The force that holds the elements together is called a chemical bond.
Covalent bonds
a chemical bond formed when electrons are shared between two atoms. example: water
Different atoms do not share electrons equally resulting in partially negative and positive regions.
For example, in a water molecule the oxygen atom has a stronger attraction for the electrons resulting in a partially negative charge.
Ionic bonds
a chemical bond that holds positive and negative ions together. In this type of bonding, electrons are not shared, they are transferred example: salt
Common Properties of Ionic Compounds
- most are solid at room temperature
- most dissolve in water
- conduct electricity when dissolved in water
- have high melting and boiling points
- are usually crystalline at room temperature
The force of attraction between molecules is called van der Waals forces. The strength of attraction depends on the size of the molecule, its shape, and its ability to attract electrons.
Water droplets form because of the van der Waals forces between the slightly positive and negative charges of a water molecule being attracted to the opposite charge of other nearby water molecules.
spiders can climb smooth surfaces because of the van der Waals forces between the atoms in the hair like structures on their feet and the atoms of the surface they are climbing.
Introducing Chemical Reactions
A chemical reaction is the process by which chemical bonds between atoms are broken and new ones are formed. In chemical reactions, substances change into different substances.
Two types of changes
Physical change
occurs when matter changes its physical properties but not its chemical composition.
example :
chopping wood
Chemical change
occurs whenever matter changes into an entirely new substance. The new substance has different chemical properties.
example:
burning wood
Chemical Equations: mass is conserved
Reactants → Products
The reaction rate is the rate at which a reactant is used up or a product is formed.
A catalyst is a substance that :
- increases the rate of a chemical reaction by lowering the activation energy
- is not used up or changed in a chemical reaction
- does not change the products of the reaction
- catalysts in living things are called enzymes
The activation energy is the minimum amount of energy needed to start a chemical reaction and change reactants into products.
Chemical Reaction
exothermic reaction
a chemical reaction that releases thermal energy
endothermic reaction
a chemical reaction that absorbs thermal energy
Exploring the Properties of Water
A water molecule is composed of two hydrogen atoms and one oxygen atom that share electrons in covalent bonds.
Exploring the Properties of Water
Polarity of a Water Molecule
Water molecule is slightly negative at the oxygen end and slightly positive at the hydrogen ends
Solubility
Water is called the universal solvent
Cohesion
A water molecule is attracted to other water molecules
Adhesion
A water molecule is attracted to molecules of different substances
MIXTURES
Mixture is a combination of two or more different substances, elements and/or compounds and can be separated by physical mean.
Homogeneous mixtures
a mixture in which the individual substances are evenly distributed throughout.
ex: solution
solute
substance that is dissolved
solvent
substance in which the solute dissolves
Heterogeneous mixtures
a mixture in which the individual substances are not evenly distributed throughout.
ex: suspension
sand and water
ex: colloid
milk / ink / paint / fog
pH Scale
pH is a measure of the concentration of hydrogen ions in a solution. The pH scale is a numeric scale used to determine whether a substance is acidic or basic, and to measure how strong an acid or base is.
Buffers are mixtures that react with acids and bases to keep the pH levels relatively stable.
Introducing the Major Biological Macromolecules
The elements that are found in greater abundance in living things are oxygen, carbon, hydrogen, nitrogen, and phosphorus. These elements are organized into larger structures called molecules.
A carbon atom has 4 electrons in its outermost energy level. This means carbon can form 4 covalent bonds with other elements.
Carbon atoms make up the backbone of many important molecules in your body like:
carbohydrates, lipids, proteins, and nucleic acids.
These complex molecules are called biological macromolecules.
are large molecules (polymers) that are formed by joining smaller organic molecules together (monomers)
General formula(CH2O)n
made up of carbon, hydrogen, and oxygen atoms combined in a ratio of 1:2:1
Two types:
- DNA
- RNA
A protein’s structure, shape, size, and function are determined by the:
number and sequence of amino acids
Fats, oils, and waxes
Lipids
Based on the carbon-carbon bonds in their fatty acid tails, lipids can be classified into:
Saturated Fats
- all carbon atoms are bonded together by single covalent bonds
- have straight chains
- are solids at room temperature
Unsaturated Fats
- have at least one carbon-carbon double bond
- have kinks in their chains
- are liquid at room temperature
Nucleic Acids
Each nucleotide is made up of three components:
1. a nitrogenous base
2. a sugar
3. a phosphate group
Basic Structure of DNA
the bases are located in the middle
the sugar and phosphate group make up the backbone of the DNA double helix
the phosphate group of one nucleotide binds to the sugar of another nucleotide
hydrogen bonds between the bases hold the two strands of the double helix together