مراجعة وتلخيص علوم منهج انجليزي الوحدة الرابعة صف سادس : نقدم اليكم في هذا الملف تلخيصا شاملا للوحدة الرابعة ، من منهج العلوم الصف السادس ،و قد تم تصميم هذا الملف لمساعدة طلابنا الاعزاء، في دراستهم و تحضيرهم للامتحان النهائي بشكل متكامل
Grade 6 Science Summary
AY 2017-2018 - Term 1
Chapter 4
The Big Idea : How does the classification of matter depend on atoms
Lesson 4.1 Substances and Mixtures
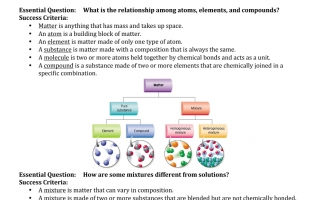
Essential Question : What is the relationship among atoms, elements, and compounds? Success Criteria
Matter is anything that has mass and takes up space
An atom is a building block of matter. An element is matter made of only one type of atom
A substance is matter made with a composition that is always the same. A molecule is two or more atoms held together by chemical bonds and acts as a unit
A compound is a substance made of two or more elements that are chemically joined in a specific combination
Essential Question : How are some mixtures different from solutions? Success Criteria
A mixture is matter that can vary in composition
A mixture is made of two or more substances that are blended but are not chemically bonded
A heterogeneous mixture is a mixture in which the substances are not evenly mixed
A Heterogeneous mixture is not a solution
A Homogenous mixture is a mixture in which two or more substances are evenly mixed, but not bonded together
A Homogenous mixture is a solution
A Heterogeneous mixture can be separated it to parts by filtering, using magnets, decanting or adding he
Essential Question : How do mixtures and compounds differ? Success Criteria
The properties of the compound are different from the properties of the atoms that make it up
mixture of compound of element x elementy elements x and y elements x and y The atoms that make up a given compound are bonded together
Changing the composition of a compound makes a new compound
Lesson 4.2 The Structure of Atoms
Essential Question: Where are protons, neutrons, and electrons located in an atom
Success Criteria
The Nucleus is the region at the center of an atom that contains most of the mass of the atom
The nucleus is made up of protons and electrons
Protons are positively charged particles in the nucleus of an atom
Neutrons are uncharged particles in the nucleus of an atom. Electrons are negatively charged particles that occupy the space in an atom outside of the nucleus. Electron Cloud is the region surrounding an atom's nucleus where one or more electrons are most likely to be found
Essential Question : How is the atomic number related to the number of protons in an atom? Success Criteria
Atomic number is the number of protons in the nucleus of an atom of an element
The identity of an atom is determined by its atomic number
Atomic 12 protons
12 proto Essential Question : What effect does changing the number of particles in an atom have on the atom's identity? Success Criteria
An isotope is one of two or more atoms of an element having the same number of protons, but a different number of neutrons
An lon is an atom that has a charge because it has gained or lost electrons
A change in the number of neutrons or electrons causes the identity of the atom to stay the same
A change in the number of protons of an atom results in a new element with a new identity